overview
OVERVIEW
About the Program
India is an innovation powerhouse, and Pfizer is committed to nurture and support ideas that can prepare the healthcare systems to be future ready. The Pfizer INDovation Program will help accelerate the journey of 14 start-ups into the market through customized support and by fostering relevant cross-industry collaborations.
Atal Innovation Mission, NITI Aayog, Department for Promotion of Industry and Internal Trade (DPIIT), Department of Pharmaceuticals (DoP), Foundation for Innovation and Technology Transfer (FITT), IIT-Delhi, Pfizer and Social Alpha have come together to launch the Pfizer INDovation Program to support breakthrough healthcare innovations by start-ups in India.
Pfizer INDovation aims to support MedTech startups developing or ready to deploy innovative, affordable, scalable, and patentable innovations that improve access to screening, diagnosis, monitoring, and treatment enablement. These technologies have the potential to transform how quality healthcare is delivered, especially in low-resource settings.
The program offers grant funding, expert mentorship, support for clinical validation and regulatory approvals, pilot deployment opportunities in public health settings, and access to an acceleration ecosystem for go-to-market readiness.
Startups can apply under one of two tracks:
- Incubation Track
Designed for startups with a clinical study-ready prototype (Technology Readiness Level 4–5), this track provides critical support for clinical validation and regulatory approvals. It enables innovators to move confidently from the lab bench to market entry—accelerating their transition toward real-world impact.
- Deployment Track
Intended for startups with market-ready products that have secured regulatory approval (TRL 8), this track facilitates pilot deployment in Ayushman Arogya Mandirs - India’s public primary healthcare centres. It supports startups in generating real-world evidence, validating clinical utility, and demonstrating measurable impact within the public health ecosystem.
eligibility



ELIGIBILITY
Who Can Apply
Start-ups may apply under one of the following two tracks, based on their product’s stage of development.
Incubation Track (TRL4/5)
- Completed pre-clinical trials or clinical equivalence studies
- Developed a process for quality assurance and quality control
- Developed a preliminary plan for clinical trials
Deployment Track (TRL 8)
- Received necessary regulatory certifications for deployment in public health
- Received necessary regulatory certifications for deployment in public health
- Willingness to travel for product demonstrations, end-user training, and feedback collection
The program will focus on high impact problem statements in areas of screening, diagnostics, monitoring and treatment enablement that have been selected after consultation with the program partners and advisors. The eligible enterprises working in any of the following areas are encouraged to apply:
Non-Communicable Diseases (NCDs)
Non-Communicable Diseases (NCDs) cause over 75% of global deaths, with India facing a heavy burden due to delayed detection and limited access to timely care.
Non-Communicable Diseases (NCDs)
- Potential solutions may include, but are not limited to:
- Point-of-care tools for effective and timely screening of NCDs that can be used by less skilled healthcare professionals or patients themselves, with minimal resources
- Tools that reduce the cost or time of specialised tests to measure disease severity, progression, or complications
- Affordable, easy-to-use platform tools in biochemistry, pathology, or imaging
Oncology
Cancers contribute to nearly 1 in 6 deaths globally, and in India, over 70% of cases are diagnosed at advanced stages, when treatment is more invasive, costlier, and less effective.
Oncology
Potential solutions include (but are not limited to):
- Accurate, easy-to-use, affordable screening tools for early cancer detection
- Diagnostic tools with timely, reliable, and gold-standard-comparable results
- Monitoring tools for relapse or metastasis, or tools that reduce the complexity, turnaround time, or cost of intra-treatment processes
Brain Health
Brain related disorders affect nearly 1 in 3 people worldwide, yet most go undiagnosed or untreated in low-resource settings.
Brain Health
- Potential solutions include (but are not limited to):
- Screening and diagnostic tools for early detection of neurological, neurodevelopmental, mental health, and substance use disorders, enabling early interventions and task-shifting
- Non-invasive or non-pharmacological treatment modalities that improve symptom management without significant side effects
- Tools to improve treatment adherence, quality of care, and reduce cost and duration of care

Maternal & Child Health
India accounts for nearly 20% of global maternal deaths and 30% of neonatal deaths, many of which are preventable with timely care..
Maternal & Child Health
Potential solutions include (but are not limited to):
- Routine and specialised screening and diagnostic tests with quick turnaround for detecting high-risk pregnancies and post-natal complications
- Tools to monitor normal and high-risk pregnancies, labour, and neonatal health in low-resource settings
- Tools for early detection of neonatal complications and timely referrals
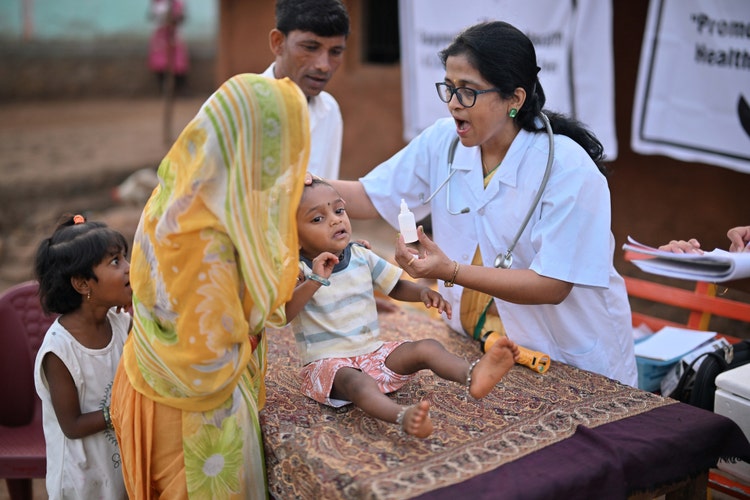
Immunisation
India vaccinates over 26 million children annually, yet timely and complete coverage remains a challenge in hard-to-reach and marginalised communities.
Immunisation
- Potential solutions include (but are not limited to):
- Energy-efficient vaccine storage and transport solutions that can run on alternative power sources
- Remote temperature monitoring solutions for last-mile delivery
- Tools to detect freezing of vaccines and prevent cold chain breaches
details
DETAILS
Timelines & Offerings

2nd September, 2025
30th September, 2025

Grand Jury
November 2025

Date to be announced shortly

Date to be announced shortly

Grant Support
Grant of up to INR 60 Lakh for clinical validation, regulatory approval and pilot deployments

Venture Acceleration
18-months engagement for curated technical and business advisory

Clinical Validation
Planning for clinical validation, trials and real-world evidence studies, access to test-beds

Regulatory Support
Guidance on product certification, regulatory approvals, patents and standards

Market Access and Scale-up Support
Facilitation of introduction to national public/private healthcare systems through partner stakeholders

FITT Incubation
Access to FITT's support for product development, or any other approved technology incubation facility

Marketing and Branding
Support for digital and print media coverage, showcasing opportunities

Business Advisory Workshops
Workshops with experts, specialists, and sector leaders for developing the product market fit and go-to-market strategy

Social Alpha Incubation
Access to renowned industry experts for mentoringand knowledge sessions conducted by Pfizer and Social Alpha

Opportunities for Follow-On Funding
Opportunity to pitch for Social Alpha's seed support and pitch to a large and diverse investor and donor network
DETAILS
Program Design




contact
CONTACT
Got Queries?
For any assistance, please reach out to us at [email protected]. Or fill the form out and we'll get back to you.
.
COLLABORATORS
Our Partners
A mission-aligned, cross-industry collaboration has come together to support the selected winners to build contextual solutions for strengthening India's healthcare system, and achieve wider market access in India, and the world.